What is a dipole moment?
They say when there is charge separation dipole moment exists i.e., either in covalent or ionic bonding. Dipole moment is usually defined as the product of the charge’s magnitude and the distance of separation between the positive and negative charges’ centers. It has a unit Debye with a direction from negative to positive.
Mathematically, it is represented as:
µ or Dipole moment = magnitude of the charge, denoted by Q distance between the positive and negative charges, denoted by r
1 Debye which is a unit of dipole moment = 3.33564 × 10-30 Cm
Where C stands for Coulomb and m represents meter.
Why Dipole moment () occurs?
It occurs as a result of the electronegativity difference between two atoms that are bonded chemically.
Example of a bond dipole moment:
Thedipole moment that exists in a bond,where there is an electronegativity difference in the atoms is termed as bond dipole moment. It is expressed as under:
or dipole moment = 𝛿. d,
Where 𝛿 is the magnitude of the partial charges.
And d is the distance between 𝛿– and 𝛿+
It is a vector quantity, with a direction parallel to the axis of the bond and magnitude. An example below is the dipole moment coming out in the case of hydrochloric acid (HCl) molecule:
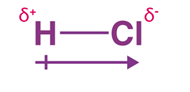
The symbols 𝛿+ and 𝛿– are the two charges that come out in a molecule that have equal magnitude, however, have the signs of the charge are opposite. The two charges are separated by a set distance, which in this example is denoted by d.
The arrow that represents the dipole moment is usually drawn from the positive and ending at the negative charge.
Another example is the Dipole moment of the Beryllium Fluoride molecule:
The bond angle is 180between the two bonds of Beryllium-fluorine. Fluorine here is more electronegative compared to Beryllium, hence shifts the density of electrons more towards it. It can be illustrated as under:
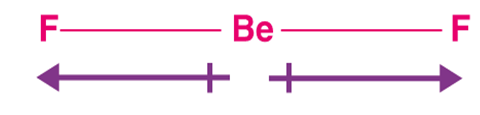

From the figure, it can be seen that the two bond dipole moments are equal in magnitude with opposite directions and hence cancel each other making the total dipole moment zero of Beryllium Fluoride.
In the case where the atoms of different electronegativities are made to interact, the more electronegative atom tends to attract the electrons or tends to move them from their initial positionsto itself. Therefore, this movement is however represented by the bond dipole moment.
Related terms where dipole moment is referred to:
- Electric : This refers to the measurement of a system’s electrical polarity of charges.
- Molecular : This refers to a molecule’s electric .
- Electron-electric : it is the measurement of the distribution of charge within a specific electron.
- Transition : This usually refers to the electrical in Physics (quantum mechanics).
- Magnetic dipole moment: This is most commonly referred to as the measure of the system of charges’ magnetic polarity
- Bond : This dipole moment is the measure of a chemical bond’s polarity.
- There is also a term known as Electron magnetic dipole moment.
- Another is the nuclear magnetic moment: This refers to the magnetic moment of the nucleus.
Brief Explanation:
- Electric dipole moment:
It is a measure of the polarity of the system of charges as a whole defined by the separation of the charges of opposite signs within the system. Its units are given by Coulomb-meter or (C·m) and are commonly written as Debye.
- Molecular dipole moment
This occurs when a molecule has two atoms of different electronegativity where one atom with more electronegativity attracts electrons more compared to the other and hence becoming more whereas the other becoming more positive.
- Electron-electric dipole moment:
This is denoted by de and is considered as a property of an electron intrinsically where the potential energy must be related linearly to the electric field’s strength given as:
U= de E
- Transition dipole moment:
This often relates to the Electric associated with the transition between an initial state{m} to a final state {n}. it is a vector quantity where the direction gives the transition polarization. Its SI unit Coulomb-meter (Cm); usually denoted by Debye or D.
- Magnetic dipole moment:
The Magnetic dipole moment is usually related to the properties of magnets or the properties of electric current loops magnetically. It is a vector quantity where it equals the product of the current flowing in the loop and the area surrounding the loop. The direction is obtained by the rotations’ right-hand rule.
- Electron magnetic moment:
The electron magnetic dipole moment is a commonly used term in atomic Physics when it comes to the discussion of an electron’s magnetic momentas a result of the electron’selectric charge and intrinsic spin properties and. The value usually comes around −9.284764×10−24 J/T of the electron magnetic moment.
- Bond dipole moment
This dipole moment has almost the same idea as that of the electric dipole moment. It is generally considered as the measure of the chemical bond’s polarity present in a molecule. It takes place only when there is a separation of charges. μ is usually given by:
μ =δ.d
where δ is the magnitude of the charges and d is the distance of the separated charge δ+ and δ–. It is a vector quantity with a direction parallel to the axis of the bond given by an arrow from positive to negative.
- Nuclear magnetic moment:
The type of moment is considered as the magnetic moment that existed in an atomic nucleus occurring as a result of the spin of neutrons and protons. The nuclei having spin not equal to zero expresses a magnetic moment not equal to zero and vice versa.